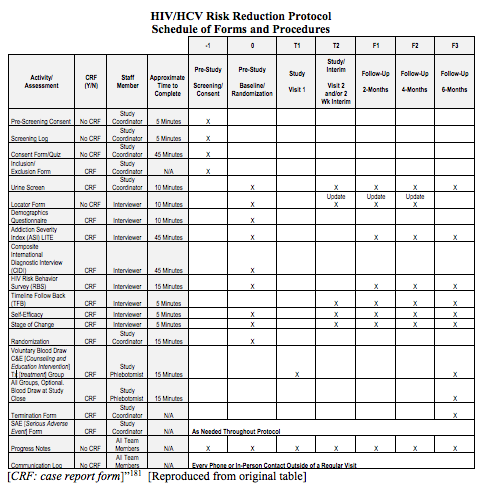
Schedule of Events. Example visit and assessment specification from a... | Download Scientific Diagram
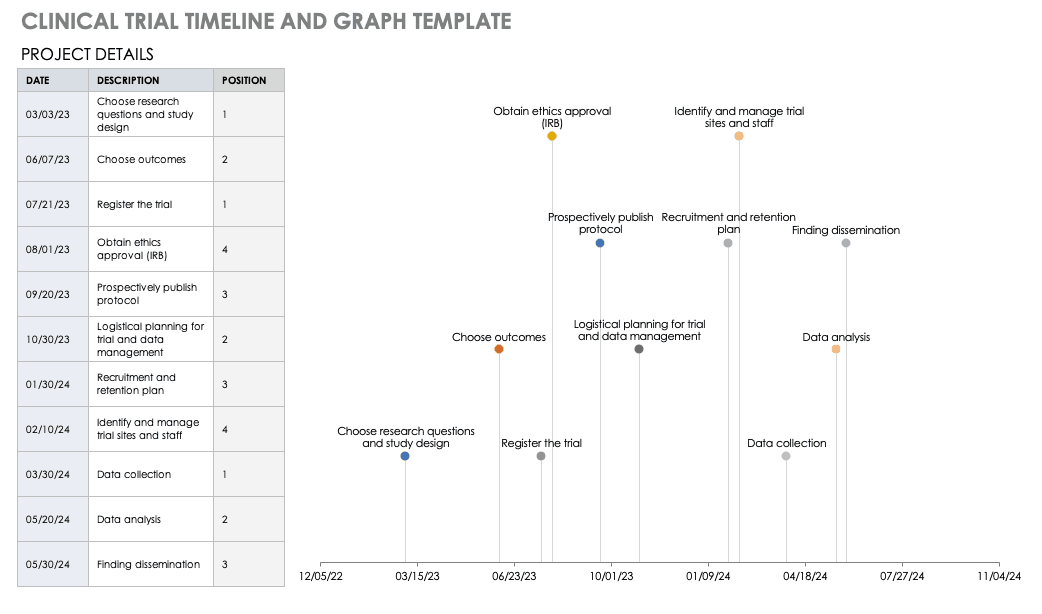
Therapeutics for Dengue: Recommendations for Design and Conduct of Early-Phase Clinical Trials | PLOS Neglected Tropical Diseases
PLOS Medicine: Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis

Table 2 from Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials)†. | Semantic Scholar

Figure 1 from Knowledge-data integration for temporal reasoning in a clinical trial system | Semantic Scholar
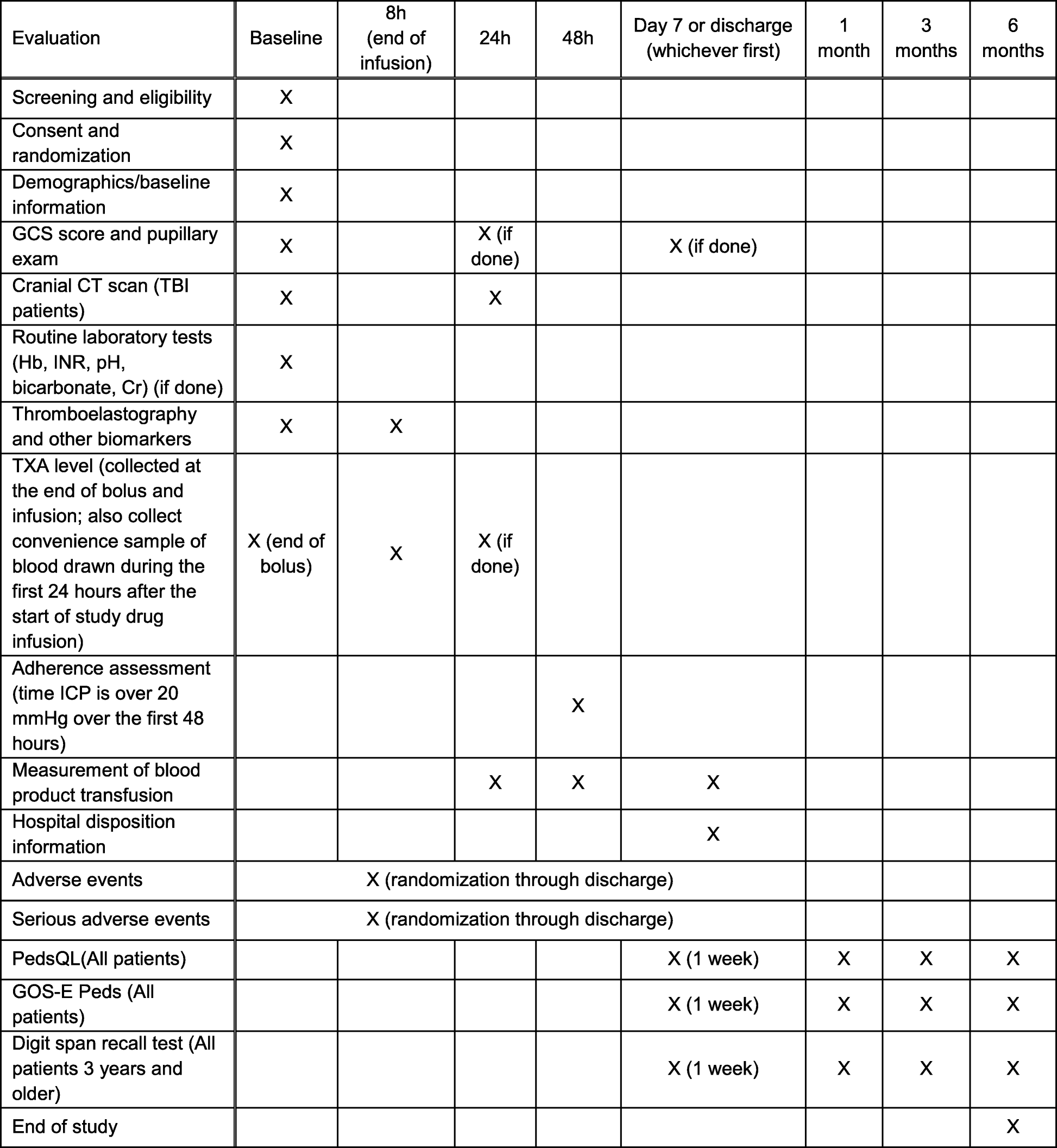
Traumatic injury clinical trial evaluating tranexamic acid in children (TIC-TOC): study protocol for a pilot randomized controlled trial | Trials | Full Text