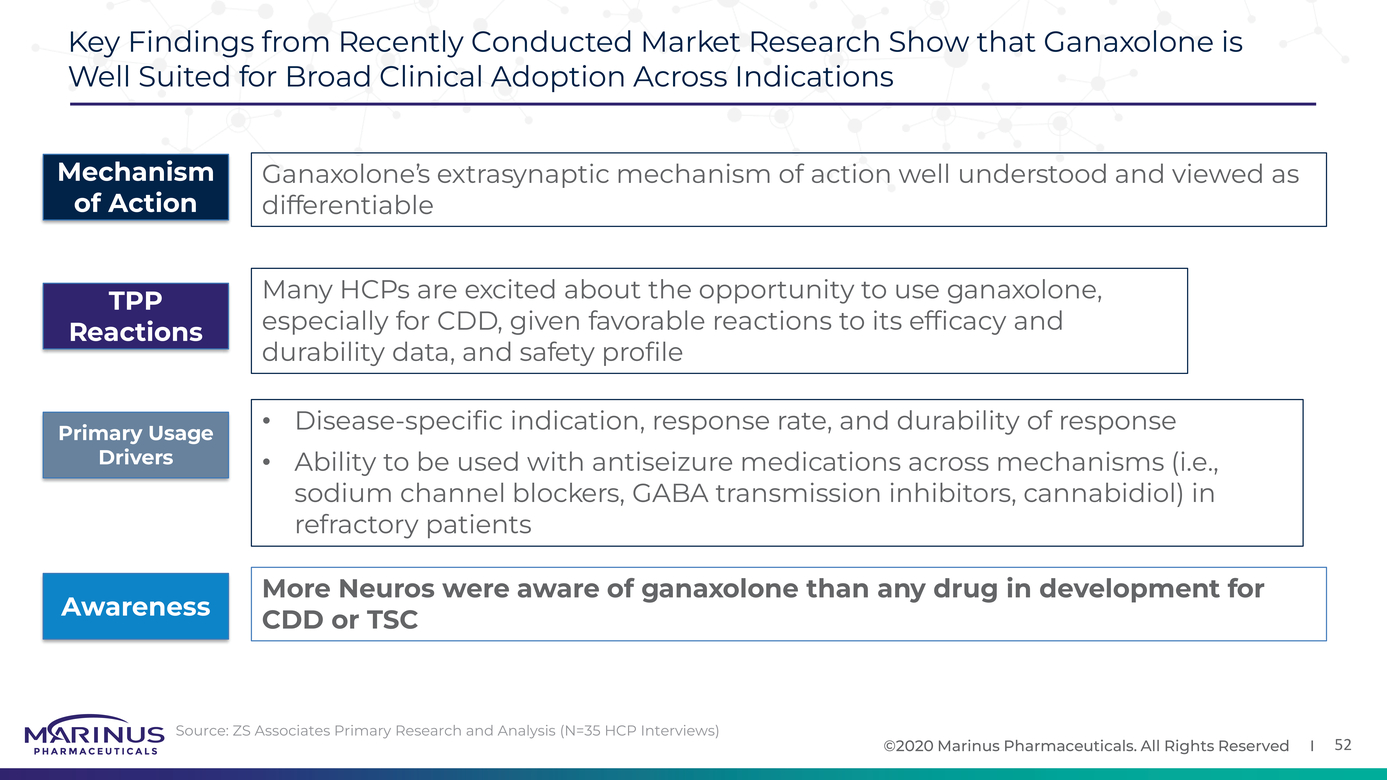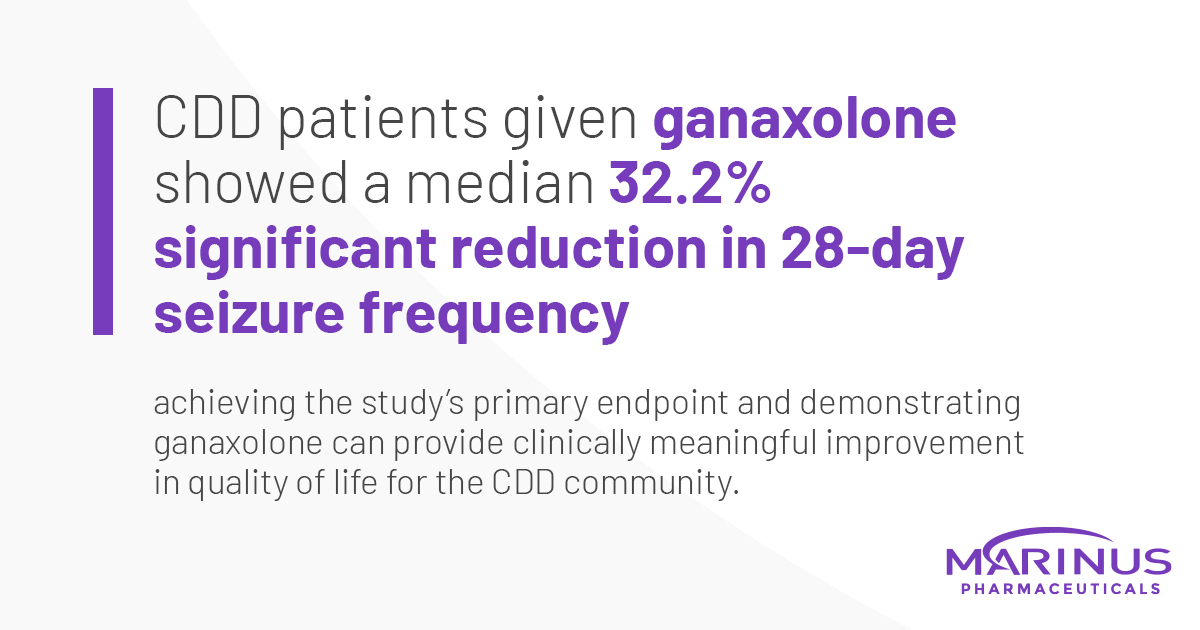
Ana Mingorance on Twitter: "Wonderful news for #CDKL5 deficiency!! Ganaxolone successfully reduces seizures in CDD (meets primary endpoint) in the first pivotal trial ever for the disorder." / Twitter
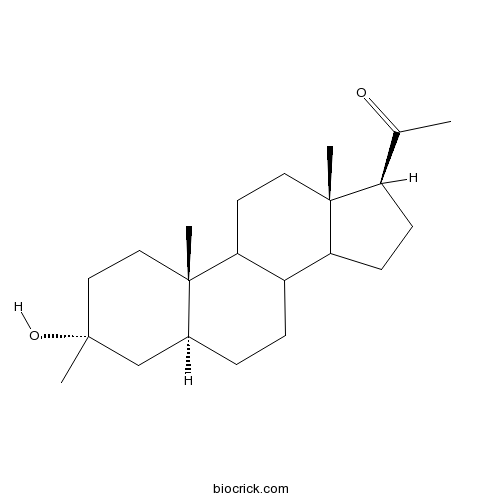
Ganaxolone | CAS:38398-32-2 | Potent, positive allosteric modulator of GABAA receptors | High Purity | Manufacturer BioCrick

Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: results from the double-blind phase of a randomised, placebo- controlled, phase 3 trial - The Lancet Neurology
Clinical Trial of Ganaxolone in Patients with Fragile X Syndrome • Fragile X Research - FRAXA Research Foundation

Ganaxolone Fragile X Clinical Trial Showed Disappointing Results • Fragile X Research - FRAXA Research Foundation

Marinus Pharmaceuticals Provides Business Update and Reports Third Quarter 2021 Financial Results | Business Wire
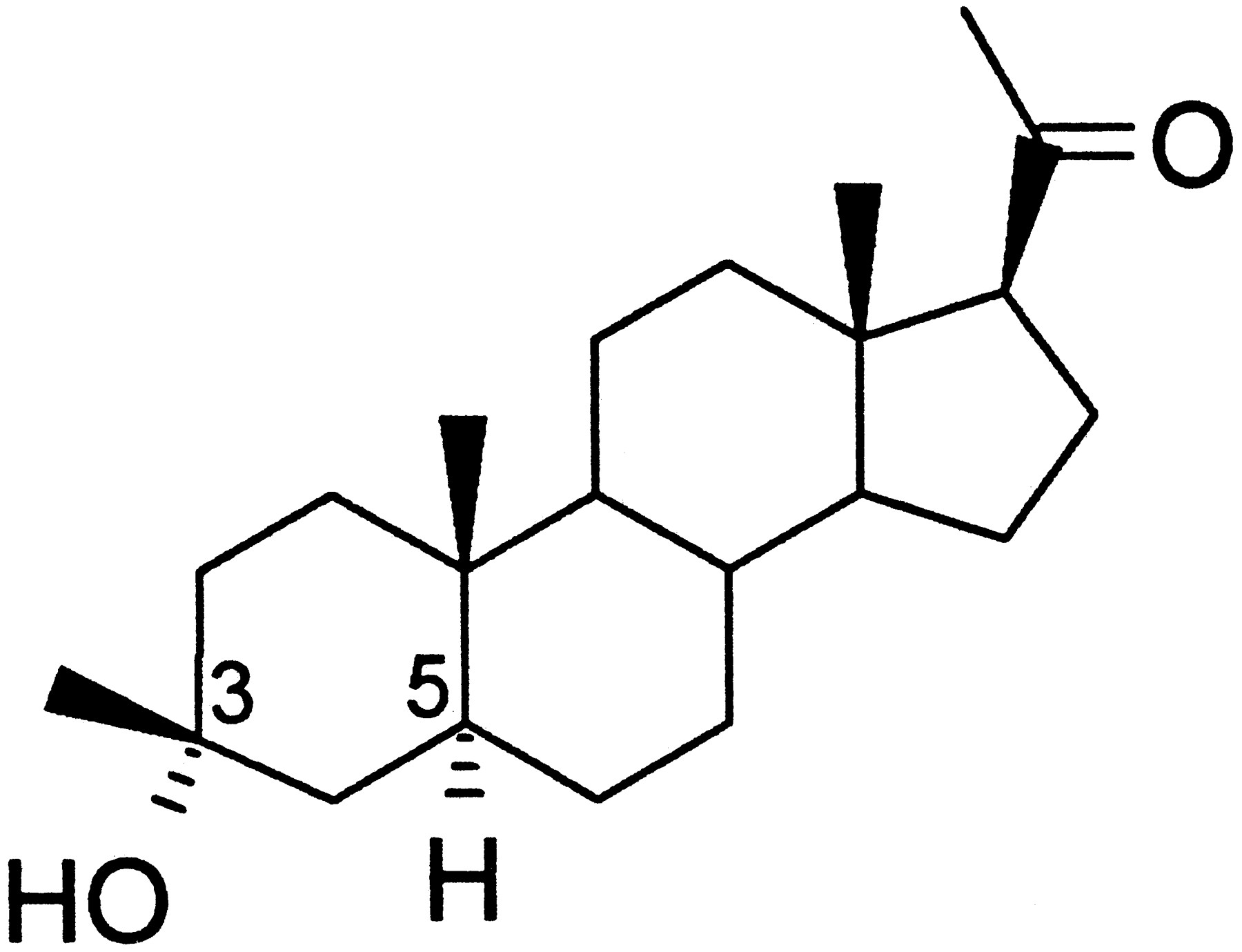
Characterization of the Anticonvulsant Properties of Ganaxolone (CCD 1042; 3α-Hydroxy-3β-methyl-5α-pregnan-20-one), a Selective, High-Affinity, Steroid Modulator of the γ-Aminobutyric AcidA Receptor | Journal of Pharmacology and Experimental Therapeutics
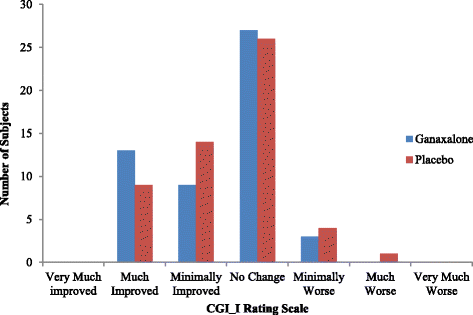
A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile X syndrome | Journal of Neurodevelopmental Disorders | Full Text

What Is Next For Investors Of Marinus Pharmaceuticals After 'Failed' Trial Of Ganaxolone In Fragile X Syndrome (NASDAQ:MRNS) | Seeking Alpha