
Chlorine azide is an inorganic compound. concentrated cln3 is notoriously unstable and may spontaneously detonate at any | CanStock
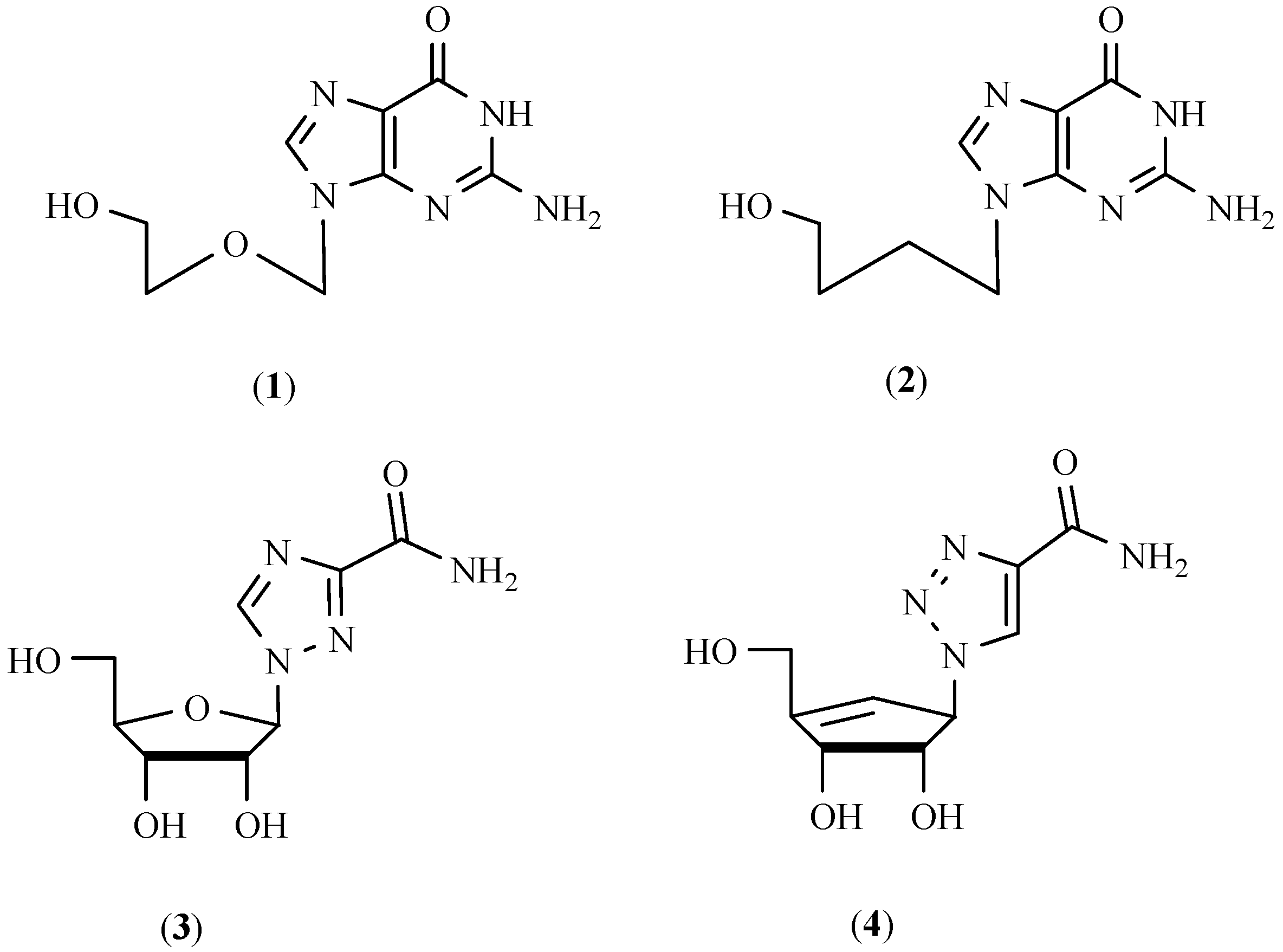
Molecules | Free Full-Text | Synthesis of 1,4-Disubstituted Mono and Bis-triazolocarbo-acyclonucleoside Analogues of 9-(4-Hydroxybutyl)guanine by Cu(I)-Catalyzed Click Azide-Alkyne Cycloaddition | HTML

Copper-azide nanoparticle: a 'catalyst-cum-reagent' for the designing of 5-alkynyl 1,4-disubstituted triazoles | Scientific Reports

Sequence-specific Labeling of Nucleic Acids and Proteins with Methyltransferases and Cofactor Analogues | Protocol (Translated to Italian)

Alkyne-Azide Cycloaddition Catalyzed by Silver Chloride and “Abnormal” Silver N-Heterocyclic Carbene Complex

Sequence-specific Labeling of Nucleic Acids and Proteins with Methyltransferases and Cofactor Analogues | Protocol (Translated to Italian)

Polimeri a spazzole contenenti triazolo ad alte prestazioni tramite azide-alkyne click chemistry: una nuova piattaforma polimerica funzionale per dispositivi di memoria elettrica - materiali npg asia

Copper-azide nanoparticle: a 'catalyst-cum-reagent' for the designing of 5-alkynyl 1,4-disubstituted triazoles | Scientific Reports

Copper-azide nanoparticle: a 'catalyst-cum-reagent' for the designing of 5-alkynyl 1,4-disubstituted triazoles | Scientific Reports

One-pot three-component synthesis of substituted 2-(1,2,3-triazol-1-yl)pyrimidines from pyrimidin-2-yl sulfonates, sodium azide and active methylene ketones

A computational study on the mechanism and the transition states of the cyclization of 1-trifluoromethyl-1,3-dicarbonyl compounds with azides to form 1,2,3-triazoles - ScienceDirect


![Metal-free 1,5-regioselective azide-alkyne [3+2]-cycloaddition. | Semantic Scholar Metal-free 1,5-regioselective azide-alkyne [3+2]-cycloaddition. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56bca88951075c37373a33573b141a738bfb34b4/6-Table3-1.png)



![Metal-free 1,5-regioselective azide-alkyne [3+2]-cycloaddition. | Semantic Scholar Metal-free 1,5-regioselective azide-alkyne [3+2]-cycloaddition. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56bca88951075c37373a33573b141a738bfb34b4/4-Figure2-1.png)


![Metal-free 1,5-regioselective azide-alkyne [3+2]-cycloaddition. | Semantic Scholar Metal-free 1,5-regioselective azide-alkyne [3+2]-cycloaddition. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56bca88951075c37373a33573b141a738bfb34b4/5-Table2-1.png)