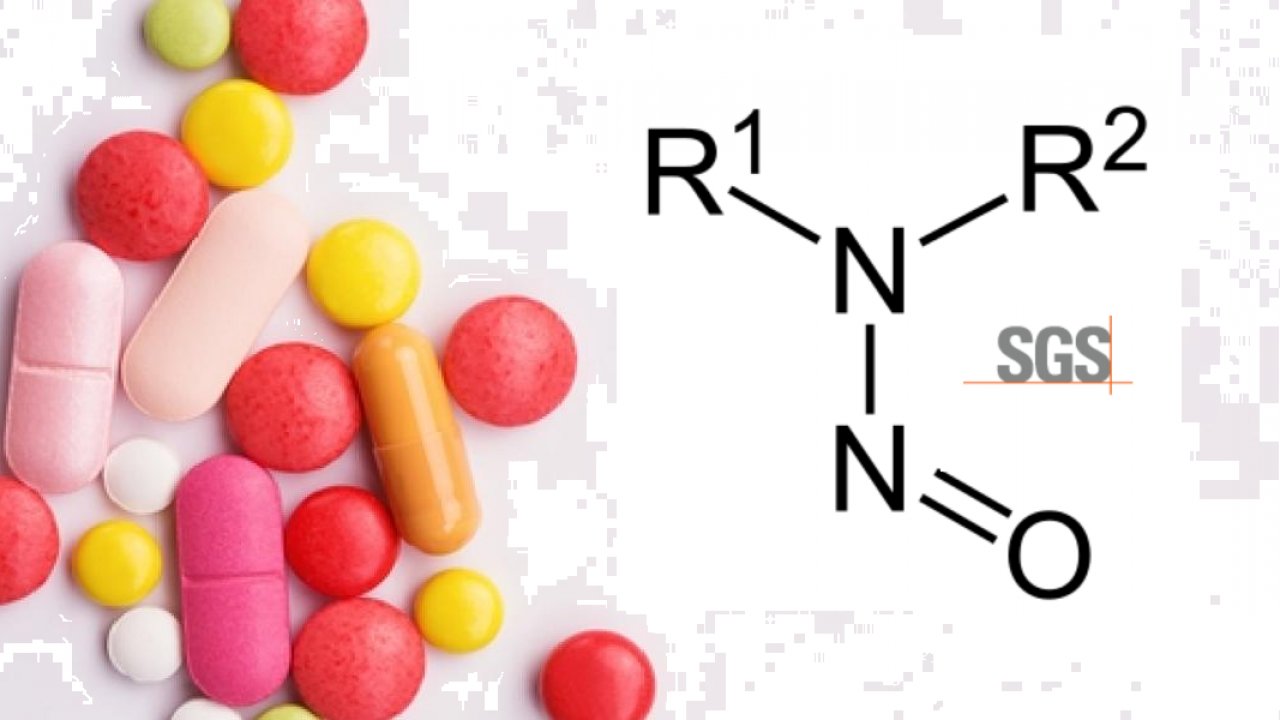
SGS unveils test method to identify nitrosamine impurities in drug products, raw materials and APIs | EuropaWire.eu | The European Union's press release distribution & newswire service

2021 News Update: Nitrosamine Impurity Testing and Analysis, including N-nitrosodimethylamine (NDMA), in API and final drug products by LC-MS/MS, GC-MS and GC-MS/MS Methods

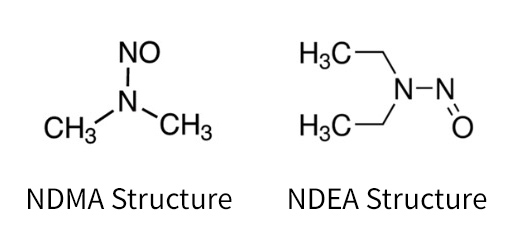
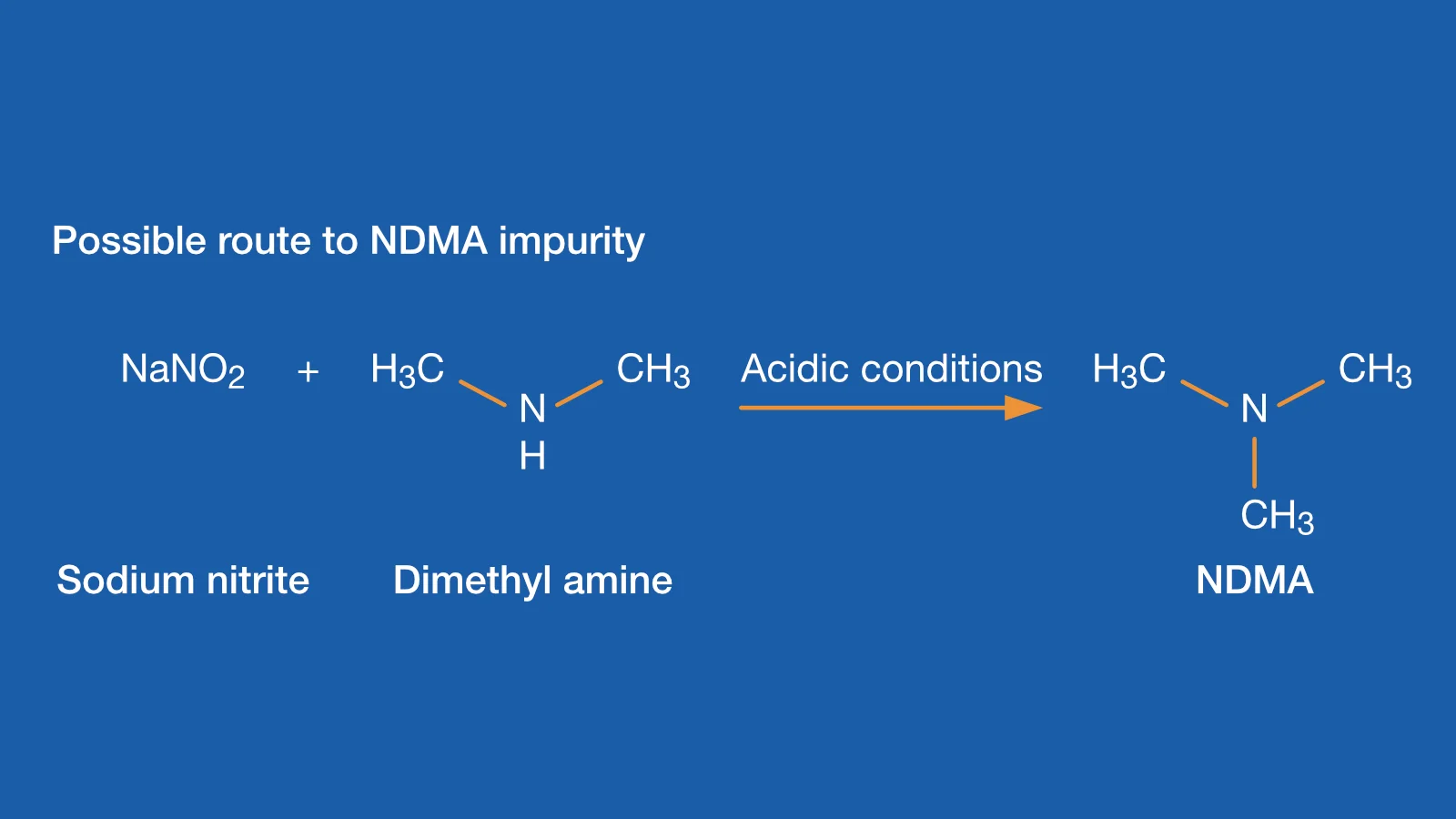
Nitrosamines in Pharmaceuticals: Toxicity, Risk Analysis, Chemistry, and Test Methods | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

Nitrosamines in Pharmaceuticals: Toxicity, Risk Analysis, Chemistry, and Test Methods | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
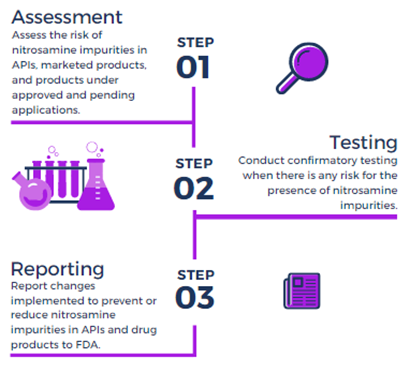
Rigorous Detection of Nitrosamine Contaminants in Metformin Products: Balancing Product Safety and Product Accessibility | FDA

European Nitrosamine Regulations - EMA/511347/2019 - FarmavitaR+ International Expert & Service Network